Determination of Sodium by Flame Emission Spectroscopy
These metals are easily excited in flames and consequently can be determined at low concentrations by flame emission. The flame photometer was calibrated with a series of NaCl standards that contained sodium equivalent to 0200400600 and 800 pg Na20 per ml.

Analysis Of Flame Emission Spectroscopy And Atomic Absorption Download Scientific Diagram
Department of Chemistry University of Kentucky CHE 226 Analytical Chemistry Laboratory 24 Na Atomic Emission EXPERIMENT 4 Determination of Sodium by Flame Atomic-Emission Spectroscopy USE ONLY DEIONIZED WATER NOT DISTILLED WATER THROUGHOUT THE ENTIRE EXPERIMENT Distilled water actually has too much sodium in it.

. The characteristic emission lines of these metals eg 5890 nm and 5896 nm for the 3p 3s transition of sodium atoms can be used in both qualitative and quantitative. Flame Atomic Emission Spectrometry of Sodium The objective of this laboratory experiment is to introduce the concept of flame emission as applied to analytical atomic spectroscopy and to explore the working concentration ranges in an air acetylene flame which is commonly used in atomic emission spectroscopy. The sodium in a series of cement samples was determined by flame emission spectroscopy.
Journal of Chemical Education v59 n10 p875-76 Oct 1982. The accurate determination of sodium content in biological samples is of primary importance in fields such as food chemistry 9 or clinical diagnosis. The purpose of this lab is to determine if the amount of sodium in the tonic water corresponds to the amount of benzoate in tonic water and to compare the amount of sodium to the amount stated on the products label.
And their application in the determination of sodium by Flame Emission Spectroscopy. Typical precision and accuracy for. 1011 Owing to its high selectivity FES usually provides for a fast and reliable quantification in agreement with clinical standards by taking advantage of the characteristic prominent yellow emission from sodium.
Sodium sample is dissolved by ultrasonic humidifier. Determination of Sodium in Salt Substitute by Flame Emission Spectroscopy. 001 nm and characteristic emission lines from the gas-phase atoms in the flame plasma the method is appreciably free of interferences from other elements.
Sodium is routinely analyzed by atomic absorbance spectroscopy AA or AAS where the sample is atomized and excited by a flame. A analytical method has been developed to determinate potassium in sodium by flame atomic emission spectroscopy. Experimental procedures and typical student dataresults are provided for an experiment determining the sodium parts per million in salt substitute with an error of.
ESTIMATIONOFSODIUMINTAPWATER SAMPLEBYUSINGFLAMEEMISSIONSPECTROPHOTOMETER 9 RESULT Thus the concentration. The flame photometer A traditional and simple method for determining sodium and potassium in biological fluids involves the technique of emission flame photometry. Because of the very narrow ca.
A analytical method has been developed to determinate potassium in sodium by flame atomic emission spectroscopy. Its most important uses have been in the determination of sodium potassium lithium and. CALCULATIONS CONCENTRATION ppm EMISSION 05 2980 1 3075 2 3655 3 3641 4 3636 5 3870 Sample concentration is 1.
The outgoing donor stream was connected to the second dialyser the acceptor stream from which was transferred on-line to the flame emission instrument for Na determination. Atomic emission spectroscopy AES employing flames also called flame emission spectroscopy FES or flame photometry has found widespread application in elemental analysis 1. As specifications for trace metals are lowered in semiconductor chemicals analysis time and the potential for contamination have increased.
The major cation of the extracellular fluid is sodium and intracellular cation found to be potassium. 45 ppm 3270 05 2980. As an analytical method atomic emission is a fast simple and sensitive method for the determination of trace metal ions in solution.
An additional goal of the continuous passive dialyser-AS coupling is automatic adaptation to analyses of samples with highly variable analyte contents which require an automatically. This relies on the principle that an alkali metal salt drawn into a non-luminous. GFAA is capable of analyzing aqueous developers with very low detection limits but GFAA is not the perfect procedure due to its long run time and the.
Sodium sample is dissolved by ultrasonic humidifier. Flame photometry is suitable for qualitative and quantitative determination of several cations especially for metals that are easily excited to higher energy levels at a relatively low flame temperature mainly Na K Rb Cs Ca Ba Cu. Determination of alkali metals in water samples.
The working conditions of the instrument and inTerferences from matrix sodium acid effect and concomitant elements have been studied. Na and K are typically analyzed by graphite furnace AAS GFAA.

Pdf An Experimental Demonstration Of A Multi Element Flame Photometer Determination Of Salt Concentration In Soy Sauce Semantic Scholar

Flame Photometry An Overview Sciencedirect Topics
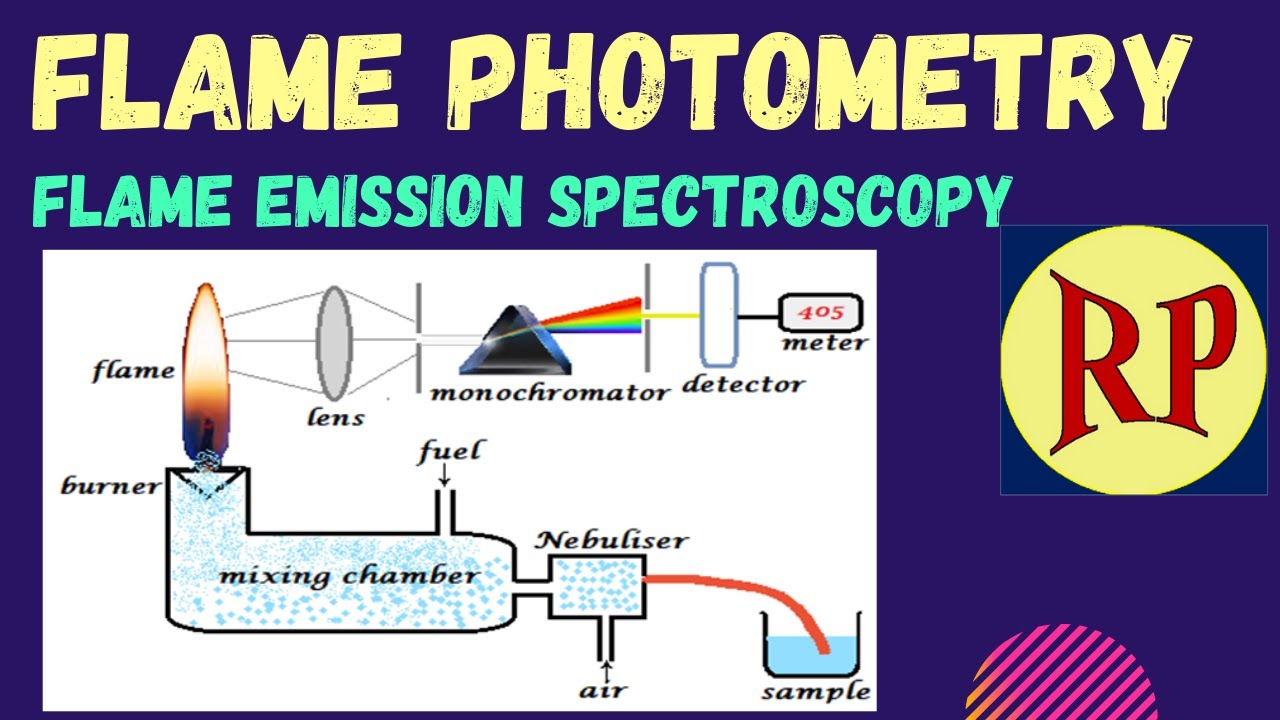
Flame Photometry Flame Emission Spectroscopy Fes Atomic Emission Spectroscopy Aes Youtube

Emission Spectra And Photographs From The Flame Tests Of Lithium Download Scientific Diagram
No comments for "Determination of Sodium by Flame Emission Spectroscopy"
Post a Comment